How do Enzyme Bring about Such High Rates of Chemical Conversion?
Definition of Enzymes: Enzymes are biological catalysts that are produced from the cell and are associated with the conversion of the substrate to another components called products. Examples are-
Reaction of enzymes: E (Enzymes)+ S (substrate)= ES complex= P (products)
Types of enzymes: Enzymes are of different types- oxidoreductase, hydrolase, lyase, lygase, isomerase etc.
Definition of Products: the formation of new substances due to chemical reactions between enzyme and substrate is called product.Examples- suppose pepsin is reacting over the substrate called protein and breaks into amino acids. Here amino acid is the product.
Definition of Substrates: substrate is the components on which the enzyme reacts. Examples- suppose lipase is an enzyme that acts on fat molecules. Here fat is the substrate.
Structure of Enzymes: As enzymes are big three dimensional molecules containing a specific pocket in itself, which is the verification specific cleft and are specific for the respective substrate binding. This pocket region of the substrate is known as active site of the enzyme.
Diffusion of Substrate Towards the Enzyme: Substrate are diffused towards the enzyme and binds with the region called active site of the enzyme. As a result an intermediate compound called enzyme- substrate or E-S complex is formed. This is an unstable complex.
Formation of Transient Compound: The complex formed due to the reaction of enzyme and substrate is known as transient compound which is formed due to the binding of enzymes-substrate. Explanation-
Release of Products: As enzyme substrate complex that formed , are converted into product.and released from the enzymes. Quantity of product can be less or more than substrate concentration.
Effect of Heat in Reaction: Enzyme substrate reaction can be endergonic or exergonic. The term endothermic means heat is absorbed during the reaction. Examples of endothermic reaction is – Endothermic reactions are also observed case of electrolysis , thermal decomposition of calcium carbonate, reaction between alcohol and sodium salt . On the other hand exothermic means heat should be given to from outside to execute the reaction. Examples of exothermic reaction is – There are many exothermic reaction, among them rusting, oxidation, titration of acid and base are the important.
Product Concentration: If the concentration of product is lower than the substrate then it is exothermic reaction means heat should be added from outside to execute the reaction.
Change of Configuration of Substrate: Whether the enzymetic reaction is exothermic or endothermic, the Substrate has to undergo a high energy transition state. And this enzyme substrate binding sometimes causes change in the structural state of substrate. Change of substrate to the products occur by altered structural state.
Breakage of Bonding: In the enzyme substrate reaction there is broken down of different bonds and re organisation of different components by bond formation is observed.
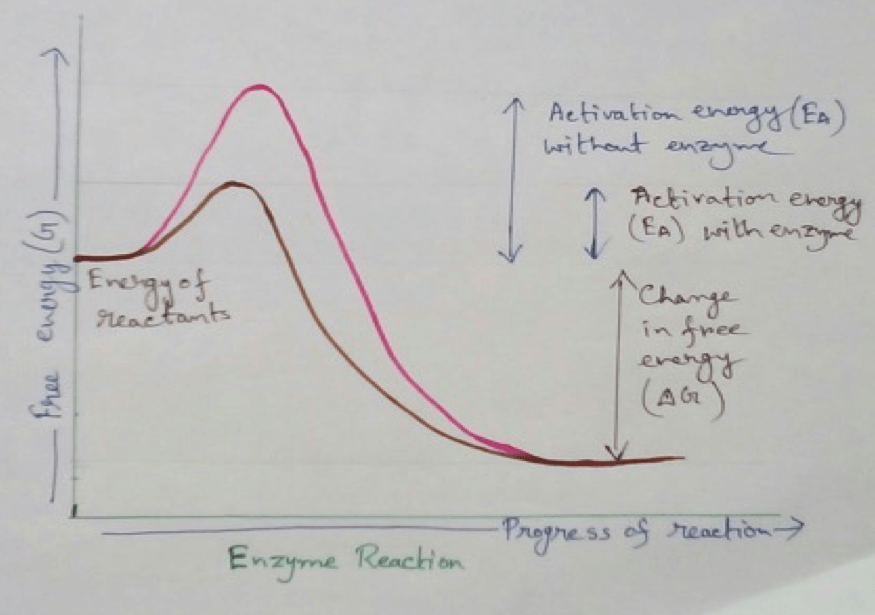
The difference between the energy content of the substrate with that of its transition state is called activation energy.
Bringing Down of Activation Energy: Enzymes bring down this high energy barrier to the lower to make the transition from substrate to the products.
FromHow do Enzyme Bring about Such High Rates of Chemical Conversion? to HOME PAGE
Recent Articles
-
Plants Around Us | Big & Small Plants | Shrubs & Herbs | Water Plants
Feb 03, 26 02:01 AM
We see different types of plants around us. Plants are living things. They breathe and grow. They also reproduce. Most of the plants grow on land. Some plants grow in water. -
Formed Elements of Blood | Erythrocytes | ESR |Leukocytes |Neutrophils
Jan 15, 26 01:25 AM
Formed elements formed elements are constitute about 45 % of blood afeias haematocrit value packed cell volume mostly of red blood corpuscles and are of 3 types- erythrocytes, leukocytes and blood pla… -
What Is Plasma? | Blood Plasma | Proteins | Nutrients | Cholesterol
Nov 07, 25 10:29 AM
Blood is a mobile fluid which is a connective tissue and is derived from the mesoderm like cell any other connective tissue. Colour of blood is reddish and that flows inside the blood vessels by means… -
Disorders of Respiratory System | Tuberculosis | Pleurisy | Emphysema
Oct 28, 25 11:39 PM
Tuberculosis is very common disease and is caused by a type of bacteria called Mycobacterium tuberculosis. This disease causes different trouble in the respiration and infection of several parts of th… -
Regulation of Respiration | Respiratory Centres | Inspiratory Area |
Oct 14, 25 12:13 AM
Respiratory Centre is the area that controls the rate of respiration and it is observed to be located in medulla oblongata and pons. Respiratory Centre has the following will dispersed components like…












New! Comments
Have your say about what you just read! Leave me a comment in the box below.