Homogeneous Solution
If there is no particle of solute left undissolved in a solution, the solution is called homogeneous solution. From a homogeneous solution no solute is obtained on filtration. The entire solution passes through the filter paper.
From a homogeneous solution, soluble solute cannot be separated from the solvent by filtration. Solvent and solute can sometimes be separated by heating the solution.
When a pan containing salt solution is heated, the water of the solution evaporates and salt particles are left behind in the pan. In slow evaporation of the water from the solution, the left behind particles of the salt have geometrical shape. These are called the crystals of salt.
When the solution of a substance is placed in a vessel and is allowed to evaporate without heating, the crystals of the solute are obtained after a few days. This way we get the crystals of substances like common-salt, alum, sugar, etc.
When an insoluble substance is dropped in water, it settles down to the bottom of the vessel containing water. The settling down of the substance in the liquid is known as sedimentation. When an insoluble substance settles down to the bottom of the vessel, the clear liquid can be poured slowly into another vessel. This is known as decantation.
From Homogeneous Solution to HOME PAGE
Recent Articles
-
Differentiation, Dedifferentiation and Redifferentiation | Definition
Apr 21, 25 01:16 PM
Cells from the root apical meristem and shoot apical meristem the camera that differentiate , mature to perform different functions. This process by which the cells undergo different major structural… -
Explain about Growth in Plants |Definition of Growth & Differentiation
Feb 27, 25 02:07 PM
Growth is a permanent increase in length or volume of an organism that brought upon by an increase in its dimensions due to synthesis of new protoplasmic material. -
Definition of Respiratory Quotient | calculation | Application | Plant
Dec 02, 24 12:09 AM
Definition of respiration quotient- the ratio of the carbon-dioxide evolved to that of the oxygen consumed by a cell, tissue, plants or animals in a given time is called respiratory quotient. It is us… -
Amphibolic Pathway | Definition | Examples | Pentose Phosphate Pathway
Jun 06, 24 10:40 AM
Definition of amphibolic pathway- Amphibolic pathway is a biochemical pathway where anabolism and catabolism are both combined together. Examples of amphibolic pathway- there are different biochemical… -
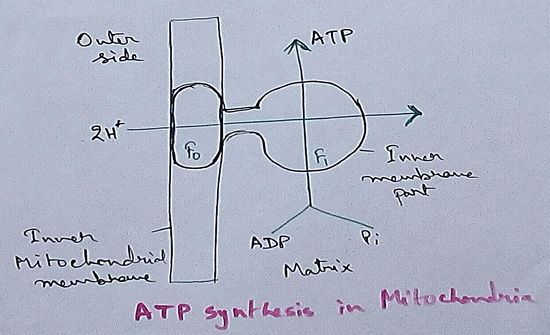
Respiratory Balance Sheet | TCA Cycle | ATP Consumption Process
Feb 18, 24 01:56 PM
The major component that produced during the photosynthesis is Glucose which is further metabolised by the different metabolic pathways like glycolysis, Krebs cycle, TCA cycle and produces energy whic…


New! Comments
Have your say about what you just read! Leave me a comment in the box below.