Explain about Enzymes
Definition of Enzymes: Enzymes are biological catalyst which speed up the reaction and it remain unchanged itself after the reaction by lowering the activation energy.
History – In 1897 Kunhe described enzyme as – “biocatalyst are proteinaceous substances which are capable of cataloguing chemical reactions of biological system without themselves undergoing any change.” In 1897 Edward Buchner first observed the fermentation of sugar, then he coined the term zymase -as it was established the participation of the zymase for complex of biocatalyst that extracted from yeast and take part in the alcoholic fermentation. This discovery gave him Nobel Prize in 1903. Sumner was the first scientist to discover the protein nature of the enzyme. In 1926 he purified the enzyme urease and obtained it’s crystalline form. For this he also got Nobel Prize in 1946. Then in 1936 Northrop confirmed the protein nature of enzyme and crystallized Pepsin and trypsin.
Types of enzymes: According to their catalytic power enzyme are of different types. They are –
Oxidoreductase: Examples are oxidase, reductase, dehydrogenase etc.
Tranferase: Causes transfer of amino group from one amino acid to other.
Hydrolase: Amylase, sucrase, lactase, maltase etc.
Isomerase: Rearrangement of molecular structure. Examples are aldose to ketose or vice versa and epimerase , mutase etc.
Lyases: Cleavagd without hydrolysis. Examples- aldolase etc.
Ligase or Synthetase: Bond formation between two by utilising energy. Example pyruvate Carboxylase.
Characteristics of the enzymes:
1. Same as catalyst, the enzymes cannot start a chemical reaction nor change its equilibrium.
2. It can enhance the rate of reaction.
3. Main role of the enzyme is to lower the activation energy or energy required to overcome energy barrier of the reaction.
4. Enzymes are produced by their respective cells they are not brought from outside.
5. Enzymes are formed in the colloidal state.
6. Particle size is almost 1-200 nm.
7. First enzymes are produced in inactive form called proenzymes or zymogen and they it converted it to active enzyme called under specific conditions. Examples are Pepsin and trypsin are auto catalytic enzyme that catalyses conversion of pepsinogen to Pepsin and trypsin oven to trypsin.
8. Enzymes those are active inside the cells are called endoenzymes and those which are active outside the cells are called exoenzymes (extra cellular or intercellular enzymes, such as digestive enzymes).
9. Primarily enzymes are called simple enzyme if they are made up of protein (Examples of protein are – Pepsin, amylase, trypsin, urease)
10. Conjugated enzyme are those protein which contains additional non protein co factor (Examples are dehydrogenase).
11. Cofactor can be organic or Inorganic.
12. Loosely attached organic co factors are called coenzyme (Examples of coenzymes are NAD,FAD, FMN,TPP, CoA)and firmly attached are prosthetic group(e.g purification group, heme, biotin etc).
13. The two groups FMN and FAD are considered as both prosthetic group and coenzymes.
14. Enzymes are having high molecular weight. Like bacteria ferredoxin having 6000, catalase having 2,50,000, urease molecular weight is 4,83,000 etc.
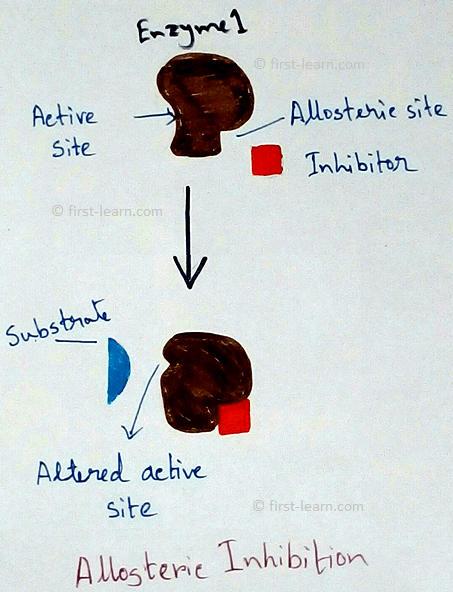
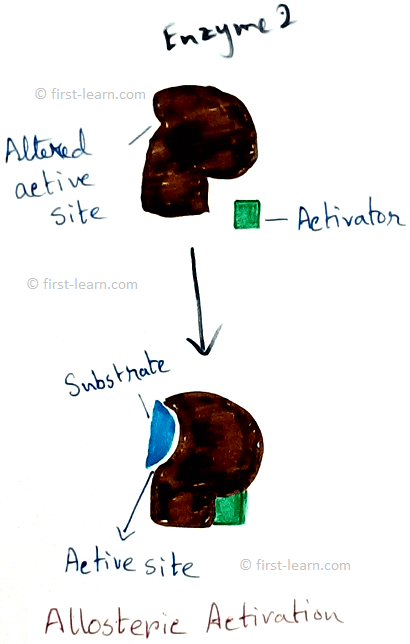
Structure and mechanism of enzyme: Each enzyme contains specific region for the catalysing activity. This portion is formed by coming together of R-groups of amino acid ,that should be 3to 12 in number. This part is called active site. The active site contains a depression in the form of crevice or pit ,where inside this the substrate molecules fit inside and undergoes some changes. Even some enzymes are also have molecular form. Examples – 16 for alpha amylase, 5 for lactate dehydrogenase. These two are called isozymes as they differ in their substrate affinities, maximum activities and regulatory properties. Some enzymes also may have areas which control the conformation of active site. These type of areas are called allosteric sites. This type of enzymes are called allosteric enzymes. Examples of allosteric enzymes are glucokinase, phosphofructokinase etc. Substances those are responsible for bringing changes in the allosteric sites are called modulators. Modulators can be of two types – positive modulators and negative modulators. Positive modulators are also called activators whereas negative modulators are called Inhibitors. Explanation of allosteric enzymes- As allosteric enzyme phosphofructokinase is activated by ADP( adenosine do phosphate) and inhibited by ATP( adenosine triphosphate phosphate). Means when ATP is broken down to ADP and inorganic phosphate that makes the activation of the enzyme on the other hand phosphate and ADP combined to form ATP that deactivated the enzyme. Totally opposite situation happens to Di phosphofructo phosphatase. Here this enzyme is activated by ATP and inhibited by ADP.
Turnover of an enzyme: As enzyme molecules are very large protein molecules in size, they remain in a colloidal state. After reaction the number of substrate molecules changed per minute is called turn over number. This is 36 million for carbonic anhydrase, 5 million for catalase, 10,000 for sucrase. The number is 50 for flavoprotein, 30 for lysozyme. This turn over number of each enzyme depends on the number of active site, rapidity of reaction and separation of end products. Each enzymes are also sensitive for substrate, temperature, pH. Each enzyme has its specific pH, temperature, substrate for their catalysing activity.
Economic Importance of enzymes: Enzymes are very useful for us. There importance in our life are-
1. Amylase, protease enzymes are added to the to the detergent for washing dishes and clothes respectively.
2. Rennut tablets that consists of rennin and chymosin are used in preparation of cheese.
3. Zymase is an enzyme secreted by yeast causes conversion of glucose into alcohol.
4. Some RNA -ase are found which have the characteristics of enzyme. It causes removal of the introns and self splicing to form the desired proteins.
5. Myosin itself is a type of protein and a type of enzyme which bears ATP in its head and associated with actin myosin interactions.
6. Digestive enzymes- Different digestive enzymes present inside our body to causes the breakdown of the food particles into water soluble and very tiny particles that absorbed by the blood. It converts all the tiny particles into simple particles.
7. Different enzymes are present inside the body associated with DNA synthesis and protein translation.
From Explain about Protein to HOME PAGE
Recent Articles
-
Plants Around Us | Big & Small Plants | Shrubs & Herbs | Water Plants
Feb 03, 26 02:01 AM
We see different types of plants around us. Plants are living things. They breathe and grow. They also reproduce. Most of the plants grow on land. Some plants grow in water. -
Formed Elements of Blood | Erythrocytes | ESR |Leukocytes |Neutrophils
Jan 15, 26 01:25 AM
Formed elements formed elements are constitute about 45 % of blood afeias haematocrit value packed cell volume mostly of red blood corpuscles and are of 3 types- erythrocytes, leukocytes and blood pla… -
What Is Plasma? | Blood Plasma | Proteins | Nutrients | Cholesterol
Nov 07, 25 10:29 AM
Blood is a mobile fluid which is a connective tissue and is derived from the mesoderm like cell any other connective tissue. Colour of blood is reddish and that flows inside the blood vessels by means… -
Disorders of Respiratory System | Tuberculosis | Pleurisy | Emphysema
Oct 28, 25 11:39 PM
Tuberculosis is very common disease and is caused by a type of bacteria called Mycobacterium tuberculosis. This disease causes different trouble in the respiration and infection of several parts of th… -
Regulation of Respiration | Respiratory Centres | Inspiratory Area |
Oct 14, 25 12:13 AM
Respiratory Centre is the area that controls the rate of respiration and it is observed to be located in medulla oblongata and pons. Respiratory Centre has the following will dispersed components like…












New! Comments
Have your say about what you just read! Leave me a comment in the box below.