Electron Transport System and Oxidative Phosphorylation
It is also called ETC.
Electron transfer means the process where one electron relocates from one atom to the other atom.
Definition of Electron Transport Chain - The biological process where a chains of redox reaction take place as electrons are transferred from the donner to the acceptor creating proton gradient pump and energy is converted into chemical energy (ATP molecules). This process takes place in mitochondria and the different energy molecules that are produced by the glycolysis, Krebs cycle are passed don this chain of reaction producing water and ATP (adenosine triphosphate).
Electron transfer with the transfer of proton creates an electrochemical proton gradient which drives the synthesis of ATP (adenosine triphosphate) which store energy chemically with a highly strainers bonds, that contain peptide, enzymes (which are protein and protein complexes.
Ultimate Electron Acceptors - In aerobic respiration electron acceptor are molecular oxygen and in anaerobic respiration electron acceptor is sulphate group.
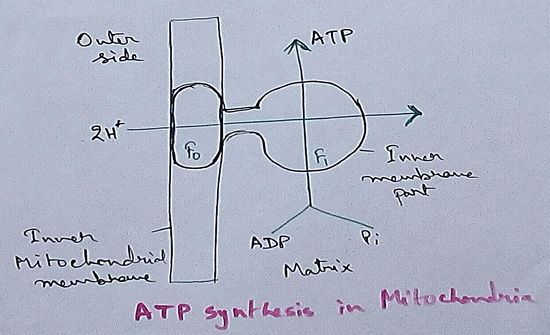
Location of Electron Transport Chain - In plants and animals electron transport chain is observed in the inner mitochondrial membrane. In eukaryotes though it is found in the inner mitochondrial membrane but it serves as the site for oxidative phosphorylation by the action of ATP synthase. In green plants especially eukaryotes it is also found in the thylakoid membrane of the chloroplasts. In bacteria electron transport chain is located in the cell membrane.
Reaction in Chloroplasts - In the chloroplasts of green plants light hydrolysis water to form oxygen and hydrogen ion then accepted by the NADP+ and converted to NADPH.
Reaction in Mitochondria - In the mitochondria oxygen is converted into water and NAD+is converted to NADH+. Conversion of succinate to fumarate and NADH+ is required for the establishment of proton gradient.
Oxidative Stress Due to Electron Transport Chain - As electron transport chain is the major site for the electron leakage thus it can create oxidative stress.
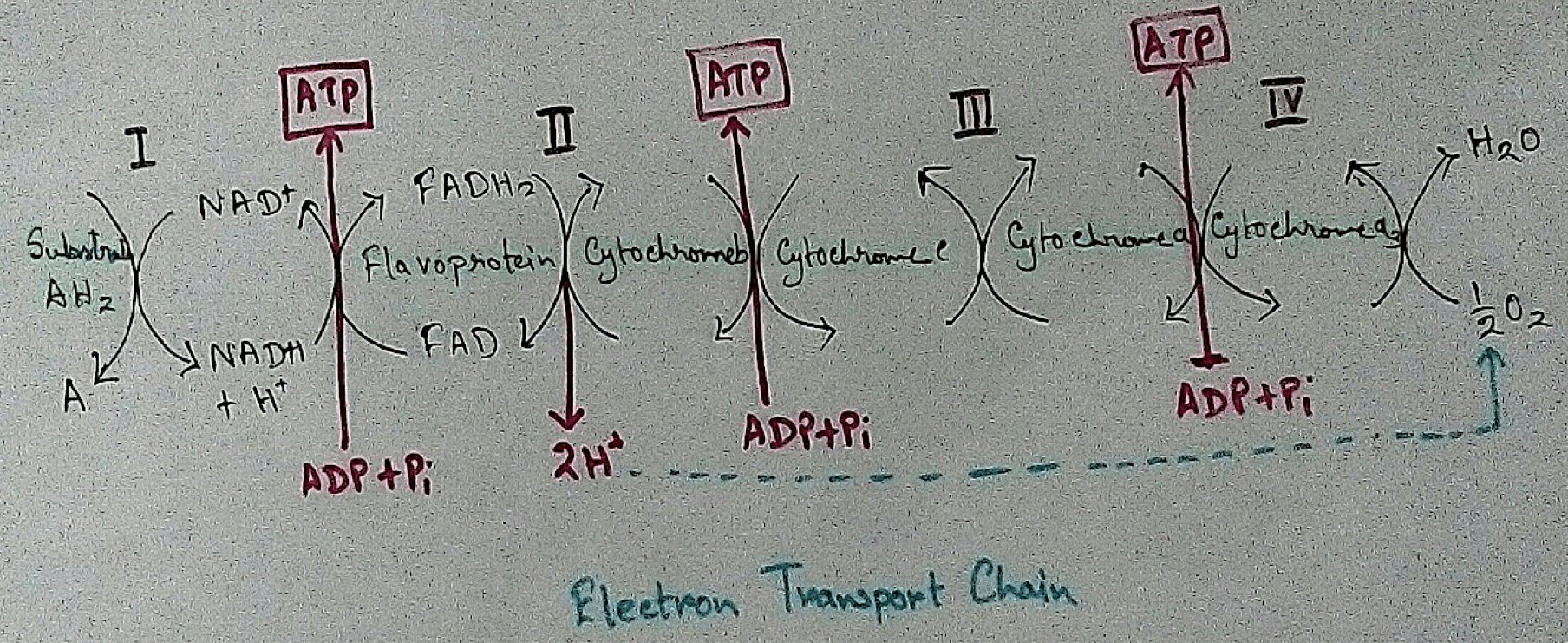
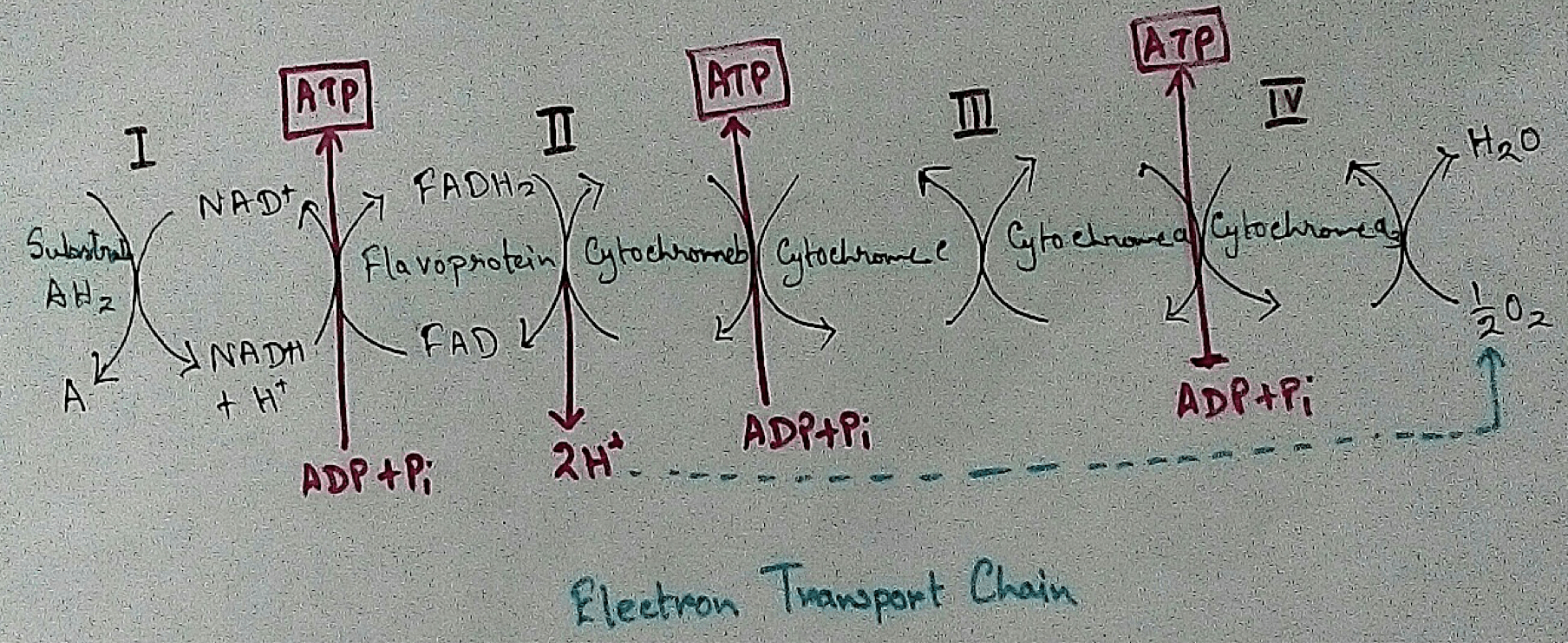
Mitochondrial Redox Carriers - Energy which is obtained due to transfer of electrons through the electron transport chain is used to pump proton from the inner mitochondrial space to the mitochondrial matrix. There are several complex that are involved in the electron transport chain.
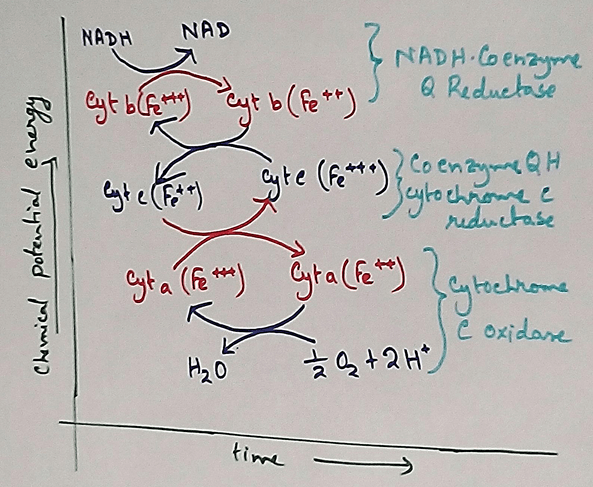
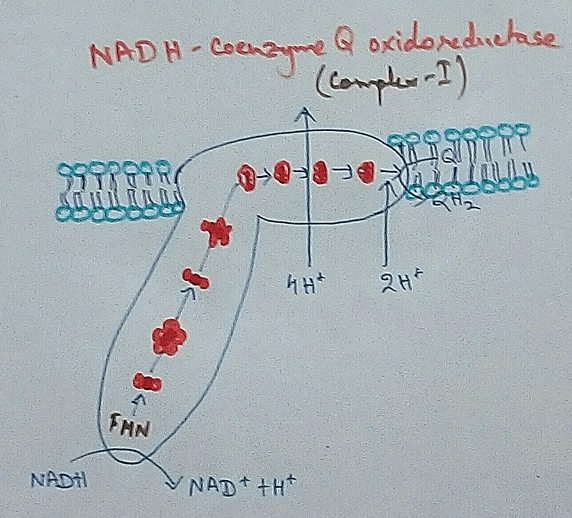
Complex 1 - NADH Coenzymes Q reductase marked as complex 1 . It is the electron acceptor of Krebs cycle. NADH (Nicotinamide adenine dinucleotide) transfer the electron into the ubiquinone that also receives electron from the complex 2.
Complex 2 – Succinate dehydrogenase marked as the complex 2. This passes electron to the complex 3.
Complex 3 - Cytochrome bc1 complex marked as complex 3 .complex 3 passes electron to cytochrome c. Then the cytochrome C passes the electron to the complex 4.
Complex 4 - Complex 4 accept electron and hydrogen ion which combined with molecular oxygen to produce water molecules. Cytochrome c oxidase is marked as complex 4.
In mitochondria already four complexes has been discovered which are membrane bound and they are embedded in the lipid layer of the membrane. Among the four complexes three are act as proton pumps and the all the four are found in the inner mitochondrial membrane. This structure is electrically connected by the lipid soluble proton pump and water soluble proton pump.
In mitochondria, electron transport chain is situated in the inner membrane where electrons move from a electron donner to the electron acceptor. Here electron donner is NADH2 or QH2 where electron acceptor is molecular oxygen that occurs by a series of redox reactions. This thing creates a proton gradient via inner mitochondrial membrane to the matrix and provides energy for pumping of the proton pump. Three complexes – 1, 3,4 are used as proton pump here. The resultant transmembrane produces ATP via ATP synthase. Reactions that occurred via complex 1 and complex 3 are almost maintained their equilibrium. It concluded that this reaction can be reversed by the change of concentration of the products and the reactants. ATP synthase can also be reversible. As a result ATP can be used for building proton gradient which are used as making proton gradient . This reverse electron transport chain is very important for some prokaryotes.
In Some Bacteria - In prokaryote like bacteria and archea have different electron acceptor and donner thus their electron transport chain is more complicated.
Bacterial electron transport chains are inducible. According to the environment bacteria can change their different electron transport chains within them by synthesing different complexes for electron acceptor and electrons donner. They select their electron transport chain from their DNA library.
In Photosynthetic Organisms - In the oxidative phosphorylation reaction electrons are transferred from a low energy compound called NADH which is an electron donner to the high energy molecular oxygen acceptor by an electron transport chains. Here high energy is used for phosphorylation reaction and this create electron acceptor and donner. Then this compounds transport electrons through electron transport chains.Here photosynthetic electron transport chain have the similarities which like the mitochondrial whee they use lipid soluble carriers Quinone and water soluble mobile cytochrome carriers. It also contains proton pumps.
Components that inhibits the electron transport chains-
Complex 1 is inhibited by rotenone, barbiturates,c hloropromazine, piercidin etc.
Complex 2 is inhibited by carboxin.
Complex 3 8s inhibited by Naphthoquinone , antimycin, British antiewistil etc.
Complex 4 is inhibited by cyanide,azide, carbon monoxide hydrogen sulphide etc.
Function of electron transport chains- There are some functions in electron transport chains. They are-
1. Acts as Proton Electrchemical Gradient - Major function of electron transport chain is to produce proton electrochemical gradient by using different proton pumps and transport electrons via the electron transport chain.
2. Conversion of Energy - If the protons flow back through the membrane they enable mechanical work like movement of flagella, cilia etc in prokaryote.
3. Highly Conserved ATP Synthase - ATP synthase has an activity that is associated with the production of ATP. This causes conversion of mechanical work into chemical energy. Thus ATP is called energy currency molecules.
4. Major Number of ATP Production - Though substrate level phosphorylation produces ATP in small amount like glycolysis. By the major number of ATP is produces by the electron transport chain.
Different complex Proteins are observed in this process for carrying the electrons and supply it to the next electron carriers. In this way high energy electrons are transported from one carrier protein to the other carrier protein. As a result water molecules are produced.
Oxidative phosphorylation:
Definition of Oxidative Phosphorylation - Oxidation phosphorylation is the metabolic pathway where cells are used oxidized nutrients by releasing energy which produces adenosine triphosphate or ATP. This is the most efficient way of releasing chemical energy than the fermentation process. This is observed in the mitochondria of eukaryotes and mainly observed in aerobic respiration.
At the time of oxidative phosphorylation electron doughnuts give electrons to the electron acceptor and this is called Redox reaction. This acceptance of electron helps in the release of high energy feet formed as ATP and released during this reaction. As we all know that ATP is the energy currency which is used by the body for different physiological activities and purpose.
Oxidative phosphorylation is executed by a series of protein complexes that are found in the intermembranous space of mitochondria for the eukaryotes and the intermembranous space of cell in prokaryotes.
From Tricarboxylic Acid Cycle to HOME PAGE
Recent Articles
-
Explain about Growth in Plants |Definition of Growth & Differentiation
Feb 27, 25 02:07 PM
Growth is a permanent increase in length or volume of an organism that brought upon by an increase in its dimensions due to synthesis of new protoplasmic material. -
Definition of Respiratory Quotient | calculation | Application | Plant
Dec 02, 24 12:09 AM
Definition of respiration quotient- the ratio of the carbon-dioxide evolved to that of the oxygen consumed by a cell, tissue, plants or animals in a given time is called respiratory quotient. It is us… -
Amphibolic Pathway | Definition | Examples | Pentose Phosphate Pathway
Jun 06, 24 10:40 AM
Definition of amphibolic pathway- Amphibolic pathway is a biochemical pathway where anabolism and catabolism are both combined together. Examples of amphibolic pathway- there are different biochemical… -
Respiratory Balance Sheet | TCA Cycle | ATP Consumption Process
Feb 18, 24 01:56 PM
The major component that produced during the photosynthesis is Glucose which is further metabolised by the different metabolic pathways like glycolysis, Krebs cycle, TCA cycle and produces energy whic… -
Electron Transport System and Oxidative Phosphorylation | ETC |Diagram
Feb 04, 24 01:57 PM
It is also called ETC. Electron transfer means the process where one electron relocates from one atom to the other atom. Definition of electron transport chain - The biological process where a chains…


New! Comments
Have your say about what you just read! Leave me a comment in the box below.